How To Find Limiting Reagent And Excess Reagent
So the excess reagent is ammonia and 575 g of ammonia will remain when the reaction reaches completion just subtract 425 from 100. Chemical reaction equations give the ideal stoichiometric relationship among.

Limiting Reactant Example Problem 1 Chemistry Problem Fuel Cells
Then subtract that from the total amount of excess reagent available.
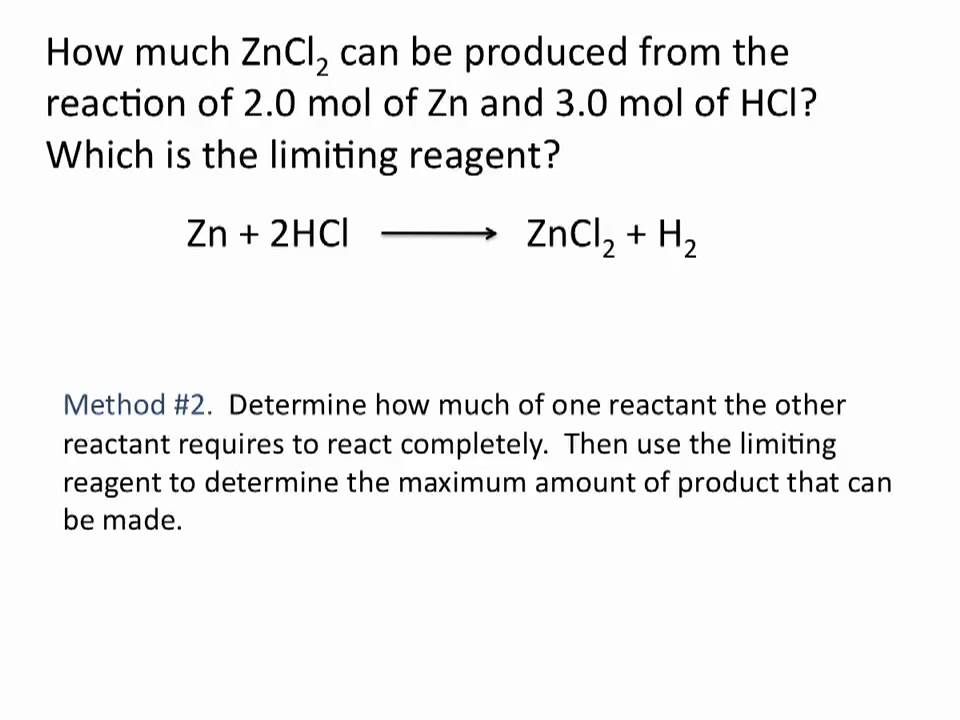
How to find limiting reagent and excess reagent. If necessary calculate how much is left in excess of the non-limiting reagent. It shows you how to perform stoichiometric calculations an. O 2 consumed in a chemical reaction can be easily determined by the comparison balanced and given chemical reactions.
A chemical reagent is a chemical species that is required in order for a chemical reaction to occur. Calculate the mass of excess reactant used up. It is called the limiting reagent.
Those are called the excess reactants. The reactant that produces a larger amount of product is the excess reagent. Introduction to Limiting Reactant and Excess Reactant The limiting reactant or limiting reagent is the first reactant to get used up in a chemical reaction.
The reactant which is earlier consumed in a chemical reaction is called limiting reactant. Determination of Excess reactant reagent consumed and un-used left over in a Chemical reaction. The following points should be considered while attempting to identify the limiting reagent.
Calculate the moles of product from the second reactant. To find the amount of remaining excess reactant subtract the mass of excess reagent consumed from the total mass of excess reagent given. Using the limiting reagent calculate the mass of the product.
This chemistry video tutorial shows you how to identify the limiting reagent and excess reactant. To determine the limiting reagent and to find out which of the reactants is in excess the stoichiometry of the reaction must be considered. Since 05 moles of O 2 react with 1 mole of H 2.
The iron is said to LIMIT the reaction. Use the amount of limiting reactant to calculate the amount of product produced. Sometimes this reagent compound is consumed during the progression of reaction but other times it is not.
The reactant that would produce the smallest amount of product is the limiting reagent. The reactant which is completely consumed in a reaction to produce product when the reaction is over is called limiting reactant. The reactant that produces a larger amount of product is the excess reagent.
The reactant that produces a lesser amount of product is the limiting reagent. All of the iron manages to react and it is this that determines the quantity of products formed. Finding the excess reactant.
2 mol H 2 needs 1 mol O 2 Balance chemical reaction. Limiting reactant is also known as limiting reagent. This calculation shows that 425 g of the original 100 g of ammonia will react before the limiting reagent is expended.
To find the mass of excess reagent find the amount of the excess reagent that reacts based on the amount of limiting reagent. In order to calculate the mass of the product first write the balanced equation and find out which reagent is in excess. Identify the limiting reactant and the excess reactant.
The limiting reagent is HCl all of the 04 moles of HCl will be used up when this reaction goes to completion The reactant in excess is Zn when the reaction has gone to completion there will be 05 - 02 03 moles of Zn left over. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Calculate theoretical yields of products formed in reactions that involve limiting reagents.
To find the amount of remaining excess reactant subtract the mass of excess reagent consumed from the total mass of excess reagent given. Main Difference Limiting Reagent vs Excess Reagent. The reactant that produces a lesser amount of product is the limiting reagent.
Once the limiting reactant gets used up the reaction has to stop and cannot continue and there is extra of the other reactants left over. 1 mol H 2 needs 05 mol O 2. Causey shows you step by step how to find the limiting reactant and excess reactant in a given reaction.
The substance whose some amount is left. Find the limiting reagent by calculating and comparing the amount of product each reactant will produce. Calculate the moles of product from the first reactant.
Balance the chemical equation for the chemical reaction. 1 mol H 2 needs 1 X 1. Use stoichiometric calculation to determine excess and limiting reagents in a chemical reaction and explain why.

Limiting And Percent Yield A Chemistry Powerpoint Lesson Powerpoint Lesson Chemistry Lessons High School Science

How To Calculate Percent Yield In Chemistry Teaching Chemistry Chemistry Physical Chemistry

Limiting Reactant Practice Problem Science Sciencewithtylerdewitt Tylerdewitt Tutor Sciencehelp School Help Online School Chemistry

Nscc Alp Chem1047 Feb 10 2015 12 12 Pm Ideal Gas Law Molar Mass Chemistry

Limiting Reagent Chemistry Tutorial Youtube Chemistry Tutorial School

Mole Conversions Made Easy How To Convert Between Grams And Moles Youtube Scientific Skills Teaching Chemistry College Words

Skillbuilder 8 1 Unit 1 Stoichiometry Math Facts Addition Chemistry Lessons Chemistry Worksheets
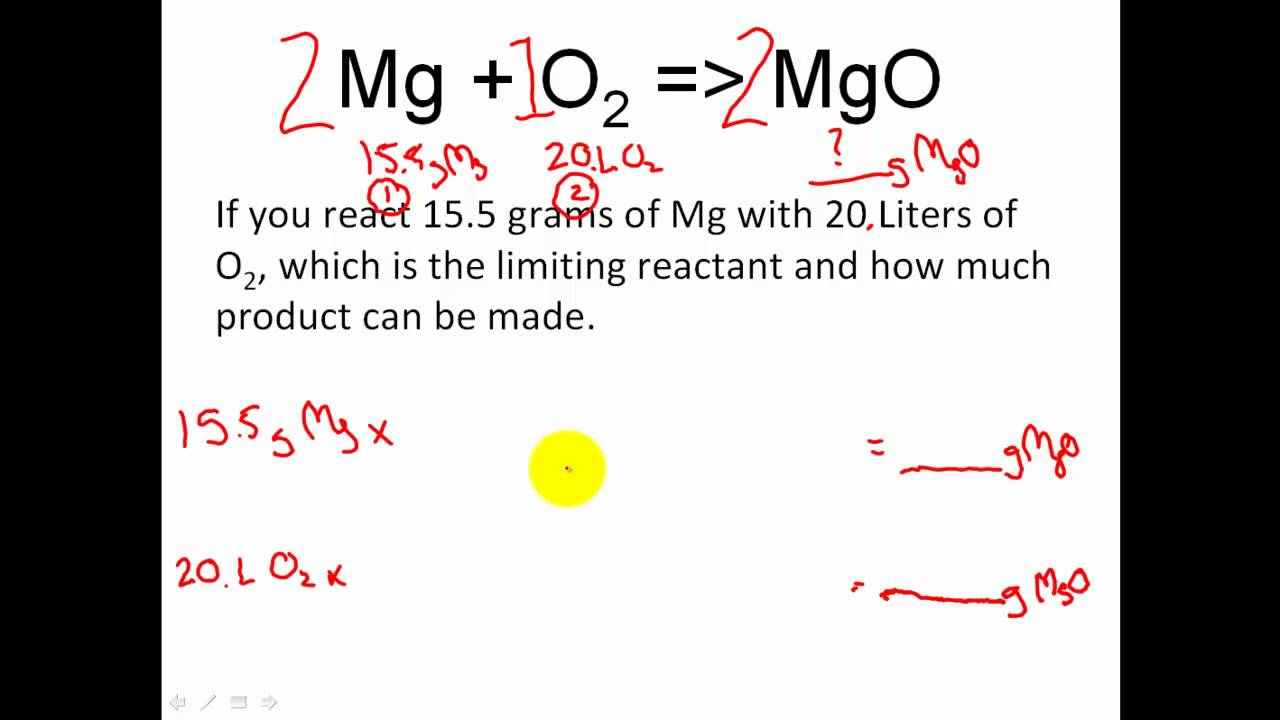
Stoichiometry Limiting Reactant Excess Reactant Stoichiometry Moles Module 6 Learning Psychology Apologia Chemistry School Work

Limiting Reactant Problems Chemistry Notes Chemistry Lecture Chemistry

Finding Limiting And Excess Reagents Chemistry Math Equations Excess

General Chemistry Educational Infographics Limiting Reactants Video Chemistry Biology Science Classroom

Difference Between Limiting Reagent And Excess Reagent Comparison Summary Teaching Chemistry Biology Notes Chemistry Notes

Theoretical Yield Chemistry Chemical Reactions Find Percentage

Balance Different Types Of Reactions With A Simple Yet Efficient Method To Help Avoid All The Confusion M Chemistry Worksheets Teaching Chemistry Mcat Study
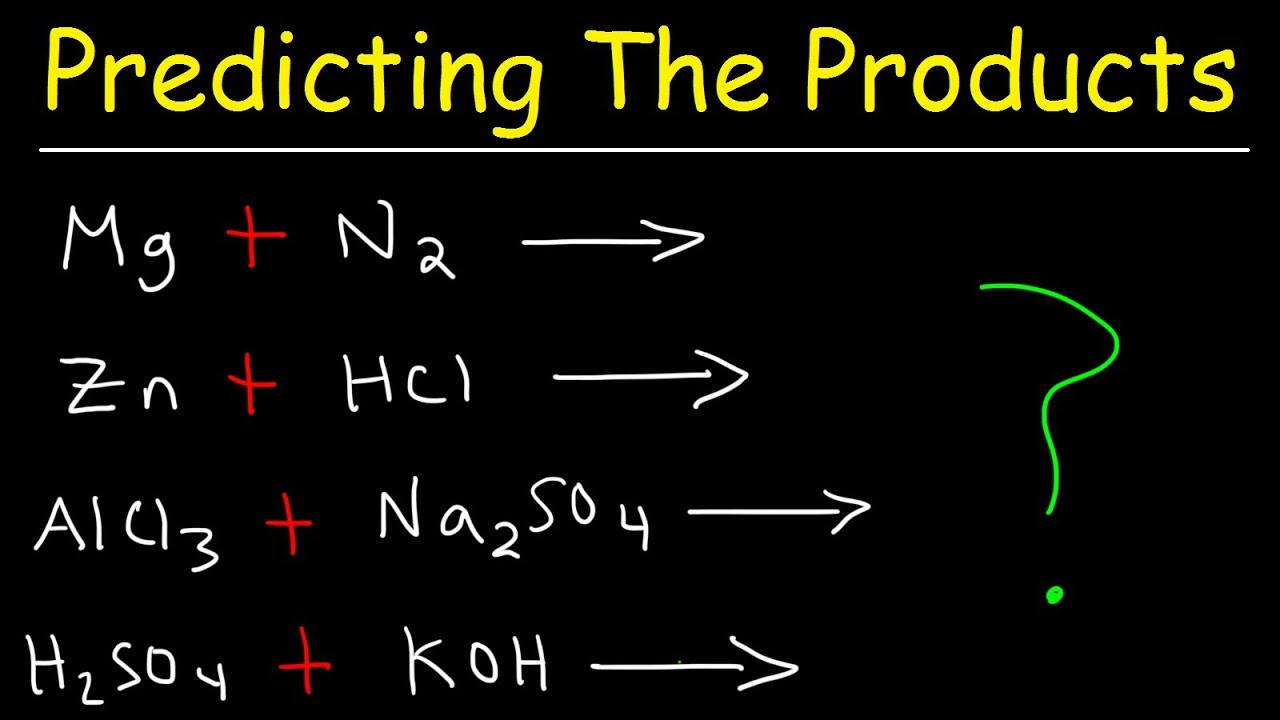
Predicting The Products Of Chemical Reactions Chemistry Examples And P Chemistry Worksheets Persuasive Writing Prompts Apologia Physical Science

Need To Calculate The Limiting Reactant Of A Chemical Reaction Chemical Reactions Chemical Chemical Equation

Limiting Reagents Grams To Grams Teaching Science Chemistry Teaching


Posting Komentar untuk "How To Find Limiting Reagent And Excess Reagent"